Mood Brightener: Here is a two minute pick me up from Stay Homas. Read their story sometime.
Here is a broad visual overview (pdf) of what we will be exploring the first 5 weeks of the course: Electrostatics Overview. You can access the original Prezi from which this was taken here.
Chemistry Review: What we know about the structure of matter
Because almost all the concepts we will explore this term involve stuff which is largely invisible to us in our daily lives and is built on our core ideas about the structure of matter this seem like a good place to start exploring.
Scale:
As we go down the scale from large to small we tend to start at the level of physical stuff. We tend to think of physical stuff as made up of lots of small parts which we can label as molecules and then atoms. Molecules being just particular collections of atoms. Atoms is the level at which we need to start digging in a little.
This term the scale of our numbers also changes. In PH211 most things are human scale in terms of positions, distances, and velocities. This term, for a variety of reasons, we will be working with numbers at (mostly) much smaller scales. A good reason to get very clear about the scientific prefixes again. Here is a link to the Bb Test you need to complete (100% to pass, multiple attempts)
Atoms:
So, typically when we start this discussion everybody is all over the idea that atoms are made of protons, neutrons, and electrons. If we had more time it would be interesting to talk about how we came to be so sure of that. Saving that for another time the next question is how you envision those protons, neutrons and electrons going together to form atoms?
Aside: Somebody always has to point out at this point that there are some other magical things called quarks that have something to do with this. That is totally true but that will need to wait until later -- thanks! If this were a face to face class I would no doubt be distracted for a while but in the remote universe I can set this question aside for later more easily!
Returning to the question of the structure of an atom no doubt you have an idea that the protons and neutrons are in a lump called the nucleus in the center of the atom. Depending on how much you've thought about it the electrons are probably visualized as small objects zipping around the nucleus in some pattern that was originally thought to be like planets (the Bohr model of the atom) and was eventually replaced with a quantum mechanical/probabilistic model. This whole probability thing is hard to visualize and it works very well to think of electrons as particles most of the time. This may not be the best place to try and sort that out just yet (like the whole quark thing!). Most of us have a mental model that we know is wrong but looks something like this....
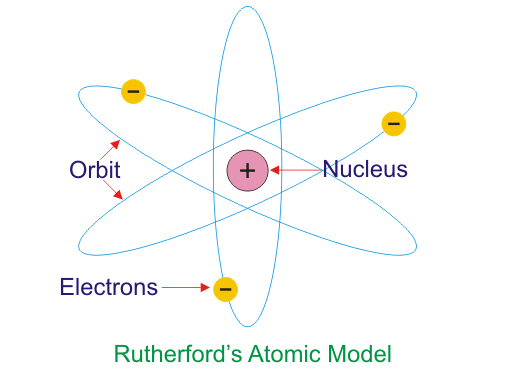
If you want to read more about the evolution of our current model and the various important experiments along the way try this physics site on atomic models. I like how it covers the topic. Be assured that I foolishly believe that ALL of the material on the previous page on atomic models is familiar and comfortable for you. Be sure and ask in class if it is not!
There are a couple of ways in which this mental picture tends to mislead us. One them is the suggestion it makes about the relative 'size' of the protons and electrons. Size is a hard concept for these sorts of objects but while we have a clear sense that protons and neutrons are about the same size, because they are almost the same mass, and we are sure the electron is smaller the question is how much smaller. In energy units (remember E = mc2? => m = E/c2!) protons are .938 GeV/c2 and electrons are 0.000511 GeV/c2. This is a ratio of 1836. In more traditional units mp = 1.67 10-27 kg and me = 9.1 10-31 kg. That's a lot more different than the picture suggests.
For class: Be prepared to identify something which is like an electron to you assume that your mass is equivalent to the mass of a proton. A burger?...a cherry tomatoe? ... a penny?
The next question of is where we tend to draw the electrons relative to the nucleus. It is important to have a general sense of the size of an atom. Generally we think of atoms as having diameters of a few angstroms. 1 angstrom = 1 10-8 cm or 0.1 nm. This diameter is a measure of where the outermost electrons are. The next part of this scale discussion is to ask about the rough size of the nucleus of an atom. It is perhaps helpful to associate fm (10-15 m) with the nucleus the same way we associate angstroms with atomic diameters. Nuclei have diameters of 2 - 15 fm. This means that, if we were drawing to scale, the outer electrons should be at a distance of 30,000 to 120,000 times the size of the nucleus we have drawn. This is clearly inconsistent with the pictures we normally draw and suggests that there is a tremendous amount of empty space in an atom.
For class: Be prepared to identify (and confirm by calculation) where something is which is the same distance away from you (as a metaphoical nucleus) as an electron?
It is an interesting question to ponder whether an atom has more empty space relative to it's parts or the solar system?
Now that we have the question of scale roughly sorted there remains the question of charge. Yup, protons have + charge and electrons have - charge and neutrons have no charge. It is important to note that the magnitude of the charge on a proton or electron is the same (1.6 10-19 C) which is why atoms are neutral. As we will discuss in class the next interesting question is whether the proton 'is' a positive charge or does it 'have' positive charge?
For class: Be prepared to describe how you could determine whether a proton has + charge or 'is' + charge. We'll talk more in the next section about why we accept only two types of charge at this time.
Given that we have found particles that behave exactly like a proton except for the fact that they have negative charge (called an anti-proton) and particles that behave exactly like electrons except they have positive charge (positrons) it would appear that charge is an attribute that can be passed to other objects like a coat. It is a bit weird but does seem to be the way of the world.
Finally, is a neutron neutral because it has no charge or because it has equal amounts of + and - charge? Atoms are neutral for the latter reason but there are other objects which are neutral because the have no charge. It had to wait for modern particle physics and the quark model to determine that a neutron is neutral because it has equal amounts of + and - charge.
Quantization:
If you only have quarters to work with there are certain amounts of money that you just can't generate. Because the charge of an electron is a finite number we can only create larger charges which are multiples of that basic charge. The charge on the electron or proton is so small that we don't typically notice this limitation but in modern electronics it is begining to be noticeable. Modern transisters in a computer only need a 100 or so electrons to switch as opposed to older version which needed 10's of thousands of electrons. With only 100 electrons doing the work a few stray electrons here or there can really mess things up. Makes the world of device electronics very interesting these days.
For a sense of scale a transistor (the basic building block of computers) can be as small as 5 nm on a side. An atom of silicon (NOT silicone the chaulking) is about 0.2 nm in diameter. That means the transistor is constructed from a few thousand atoms which is remarkable.
Note: Normally the "For class:" questions would be questions to answer before the next class via a Bb quiz or assignment.
Before Next Class:
Assignment HW: Bb Assignment
Bag 'o Water
Assuming a human being is a 70 kg bag 'o water (H2O) with some mineral contaminants how many protons are there and how much charge does this represent? Be within 20%. You will need to use a range of skills from your chemistry background to accomplish this including the definition of a mole, Avogadro's number, and understanding the periodic table.
Assignment HW: Bb Test
Adding electrons
A metal sphere has a net charge of +4 µC. Then 6.0.1013 electrons are added to the sphere. What is the net charge on the sphere after the electrons are added? Remember the charge on a single electron is 1.6.10-19 C.
Looking Ahead:
Look ahead to the next Breadcrumb: Tape and Balloons
Assignment Breadcrumb Reading: Bb Test
Occam and Razors
Who was Occam and what is his razor?
