Purpose:
We've been talking about light and colors and spectra in the classroom portion of the course but nothing beats a little hands on experience. I hope when we're done with this you'll look at colors and rainbows a little differently.
If you're doing this lab remotely and don't have access to a spectrometer do this remote version of the lab.
Procedure:
- Operation of a Spectroscope: To begin with we need to make sure you know how to use the tool which allows us to observe the different colors of light that are present in a particular source. These low cost spectrometers were developed with public funds through Project STAR. Here is a description of how they work. Explore various sources of light in the lab/room until you are confident that it is working correctly. What are the units on the scale that you can see in the spectrometer?.
- Now that you are comfortable using the spectrometer use it find two sources of light (you are advised to go outside as part of this exploration) that produce a continuous spectrum with no bright or dark lines. What is a typical characteristic of the source that produces a continuous spectrum that we talked about in class?.
- In the room will be a number of glowing 'neon' tubes filled with different gasses. Sketch, on the blank spectra provided (a separate sheet but an example is embedded below), where the bright lines are for each element (label the sketches with the element of course). Which element is the simplest and which is the most complicated? Does this correlate with the complexity of the spectrum you observe? What does a more complex spectrum look like?
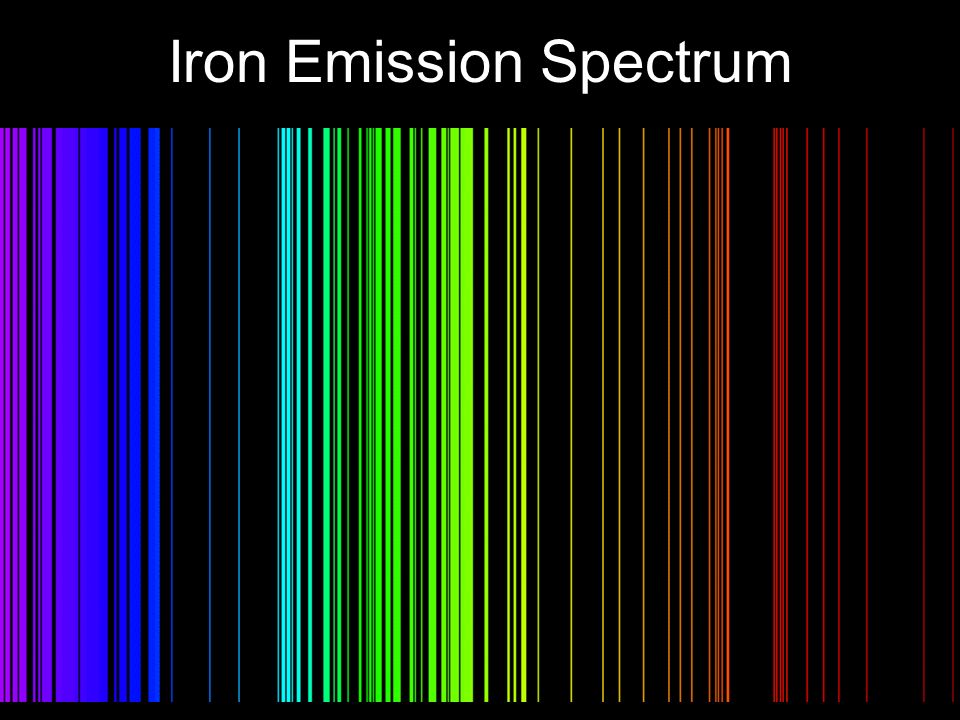
- Now turn your spectrometer on the flourescent lights in the room. Sketch the spectrum you observe and describe it verbally. Based on previous observations would you expect the element in the lights to be more or less complicated relative to the other elements you have observed? Why? How might you explain the continuous part of the spectrum? Are flourescent bulbs hot? Here is the emission spectrum for iron for comparison.
- Further explorations: Now that we have some sense of how this can be a tool for identifying some of the elements in a source of light take your spectrometer and observe other sources of light. For sure we will look at the projector light and the shop lights. I will hope to have some other sources of light for you to look and and consider. You will asked to explain what you observe about the different light sources and what you think it means.
LAB DELIVERABLES: (Turn in on Bb)
I) Which sources of light gave a continuous spectrum? This type of spectrum is typical of what sort of source that we have talked about in class?
II) Present your observations of the bright line spectra of the different elements. Discuss any correlation you observe between the complexity of the element and the complexity of the spectra. Be sure to explain what you mean by complexity in each case.
III) Present your observations of the spectrum produced by the flourescent lights. How does the complexity of this spetrum compare with those you have previously observed. Where would you place the primary element in the flourescent lights on the periodic table and why? How do you explain the continuous portion of the spectrum?
IV) Present your observations for at least two other light sources and what their spectra tell you about the elements that are producing the light?
