Purpose:
We've been talking about light and colors and spectra in the classroom portion of the course but nothing beats a little hands on experience. Because this is a remote version of this experience you will miss out on the actual experience of holding and using a spectrometer. I debated having you make one but general success might have been difficult. Instead I am using images made with a cell phone looking through our spectrometers for you to look at. Much thanks to Zelda Zeigler and Ben Tabor for developing these resources. I hope when we're done with this you'll look at colors and rainbows a little differently.
Procedure:
- Operation of a Spectroscope: In the f2f lab we have low cost spectrometers that were developed with public funds through Project STAR. Here is a description of how they work. In general we would spend some time actually figuring out how to work the beasts and making sure they were set up correctly. What are the units on the scale that you would see in the spectrometer?

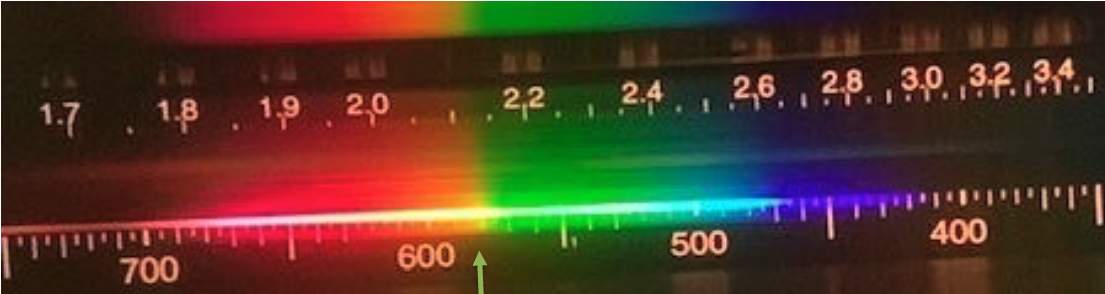
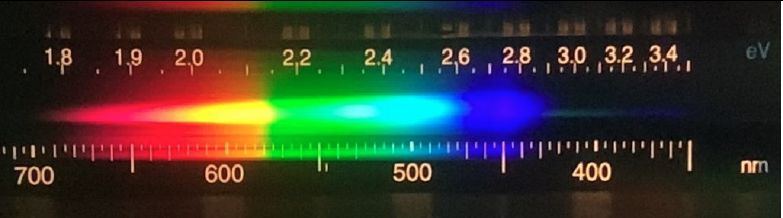
- Here is an example of a typical spectra that you would see when looking at an incandescent light bulb. Would you identify this as a bright line, dark line, or continuous spectra? What is the wavelength in nm of the yellow light indicated by the arrow?
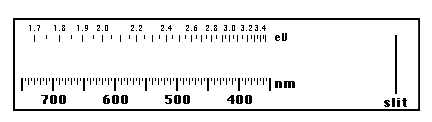
- In the room will be a number of glowing 'neon' tubes filled with different gasses. Sketch, on the blank spectra provided provided below, where the bright lines are for each element (label the sketches with the element of course). Which element has the 'simplest' spectrum and which is/are the most complicated? Look up each element on the periodic table and see what it's 'number' is. Is there a correlation between the atomic number and the complexity of the spectra? Nitrogen is more complex because it is actually a molecule of N2 which has more options than N alone.
Hydrogen:

Neon:

Nitrogen:


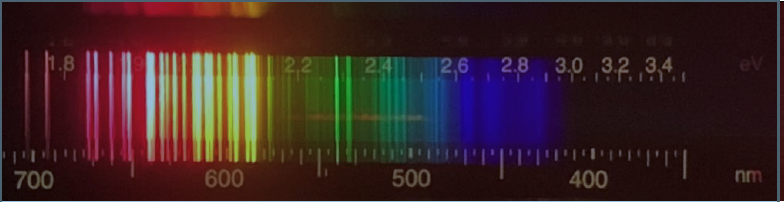
Mercury:

- Now turn your spectrometer on the flourescent lights in the room. Sketch the bright lines that you can observe. You will notice there is also a continuous part of this spectrum as well. Do the bright lines match up with any of the previous spectra you have 'observed'?
Fluorescent Light:
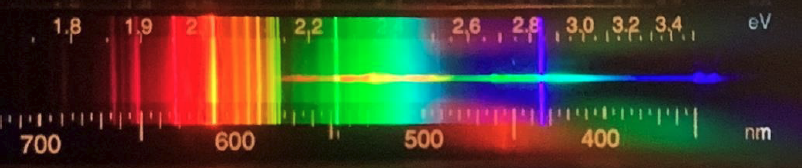

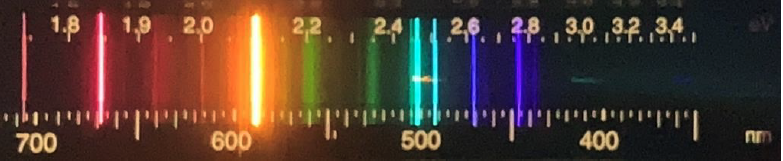
- Here is a spectrum from an LED light that we can get in the store these days. Are there bright lines in this spectrum? What are similarities and differences between this spectrum and that for the incandescent bulb?
LED:

LAB DELIVERABLES: (Turn in on LMS)
I) Complete the worksheet linked below and turn in on the LMS.
